🧪 Media Preparation — From 50 L to 1000 L
We provide reliable and scalable media preparation services ranging from 50 L to 1000 L. Our team handles full workflow execution, including powder hydration, sterile filtration, pH and osmolarity check, and in-line quality checks to ensure batch consistency. Media can be prepared as basal, supplemented, or custom formulations tailored to your cell line and process needs. This service supports both R&D and production-scale runs, ensuring you have high-quality media ready for inoculation at any stage of your bioprocess.
Flexsafe Pro Mixer — Media & Buffer Preparation
The Flexsafe Pro Mixer is widely used for the preparation of culture media and process buffers because it can dissolve powders quickly, efficiently, and uniformly. Its magnetic levitating impeller creates strong, consistent mixing without generating high shear, which helps powders such as salts, amino acids, glucose, or complex media components dissolve completely. The single-use bag system prevents contamination and eliminates cleaning steps, making it ideal for a fast turnaround. Thanks to its controlled mixing speed and efficient bottom-mounted impeller, it ensures homogeneous solutions, and speeds up buffer or media preparation across a wide range of volumes.
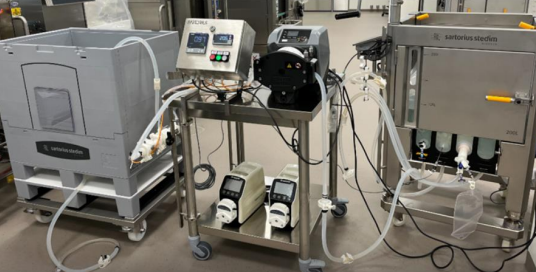
Filtration system
A filtration system equipped with a pump and disposable tubing is used to sterilize liquids during bioprocessing. The pump provides a controlled and steady flow, pushing the product through the filter while avoiding excessive pressure that could damage cells or sensitive molecules. The single-use tubing and filter capsules reduce contamination risk and eliminate cleaning steps, making the setup ideal for GMP operations.
Aliquoting Into Smaller Bags
Aliquoting consists of transferring a large bulk volume—for example, a 400 L batch of media or buffer—into smaller single-use bags such as 50 L units. Using sterile tubing and a pump, the product is dispensed into each bag under closed, contamination-free conditions. This approach improves batch management, storage flexibility and process workflow, allowing operators to handle smaller, more convenient volumes for downstream steps. The use of pre-sterilized, single-use bags eliminates cleaning and reduces cross-contamination risks, while ensuring that each 50 L portion maintains the same quality and homogeneity as the original 400 L bulk.